Electrode
An electrode is a metallic conductor with an interface to a non metallic medium (vacuum, electrolyte, semiconductor).
In electrochemistry three types of electrodes are needed to work with a potentiostat: Working, Counter and Reference Electrode. These are abbreviated with WE, RE, and CE. In most cases the electrode will have a metal-electrolyte interface.
The working electrode is the electrode where the reaction to study is taking place. In corrosion research the working electrode is usually the sample.
The reference electrode provides a constant potential. The set potential is applied between the working and reference electrode, so the potential of the working electrode is always known. To keep its potential constant, no current should flow through the reference electrode.
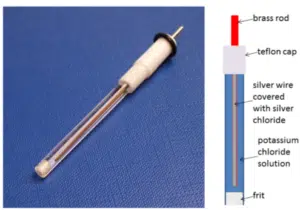
The current will flow through the working and the counter electrodes. The potential of the counter electrode is adjusted, so it provides the current demanded by the working electrode.
Articles
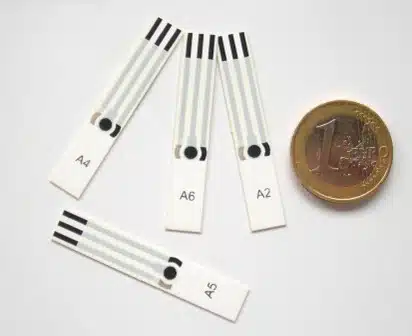
Screen Printed and Film Electrodes
Screen printed and film electrodes are suitable for mass production, versatile, cheap, and easy to handle. They make electrochemistry available to users without electrochemical knowledge.

Electrodes used with a potentiostat
This section gives a short overview of the three types of electrodes (working, reference, counter electrode) you will encounter while using a potentiostat. It is explained how these electrodes look like and what their task is.
