Luggin capillary
The Luggin capillary is a tool to move the reference electrode very close to the working electrode as described in this article.
The Luggin capillary is broad enough on one end to insert the reference electrode. The other end is a very narrow tube (capillary). The Luggin capillary is put into the electrochemical cell and filled with a supporting electrolyte or the test solution.
The narrow opening of the capillary has two advantages. It provides a clear measurement point for the reference electrode where the solution potential is measured. This way the point of the reference potential is closer to the working electrodes. A shorter distance to the working electrode means a smaller uncompensated resistance and thus a smaller IR-drop.
The second advantage is that the steric hindrance by the small capillary tip is very small.
Articles
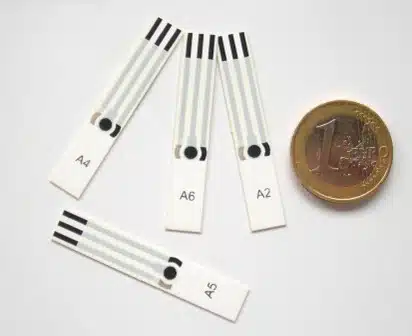
Screen Printed and Film Electrodes
Screen printed and film electrodes are suitable for mass production, versatile, cheap, and easy to handle. They make electrochemistry available to users without electrochemical knowledge.

Electrodes used with a potentiostat
This section gives a short overview of the three types of electrodes (working, reference, counter electrode) you will encounter while using a potentiostat. It is explained how these electrodes look like and what their task is.
